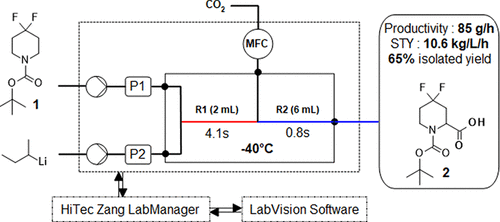
Scale-Up and Optimization of a Continuous Flow Carboxylation of N-Boc-4,4-difluoropiperidine Using s-BuLi in THF,Organic Process Research & Development - X-MOL

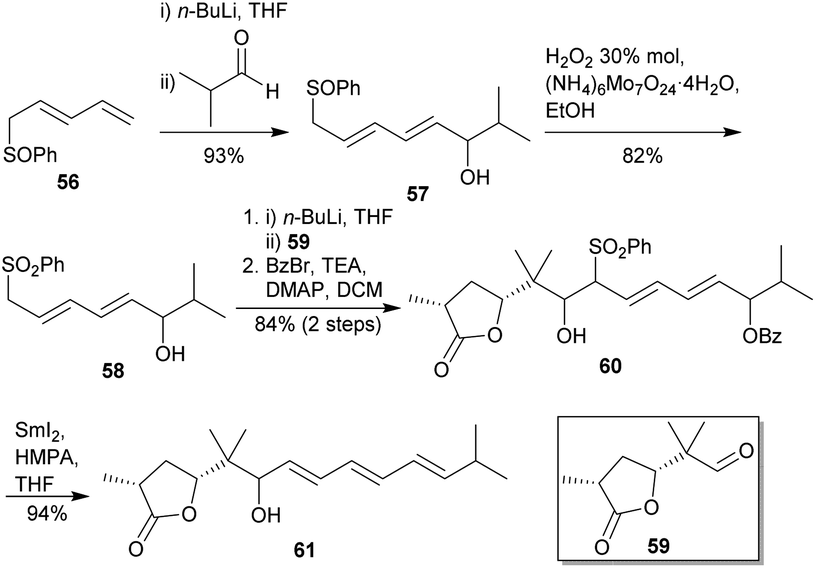
PDF) Synthetic Approach to the Core Structure of Oleandrin and Related Cardiac Glycosides with Highly Functionalized Ring D
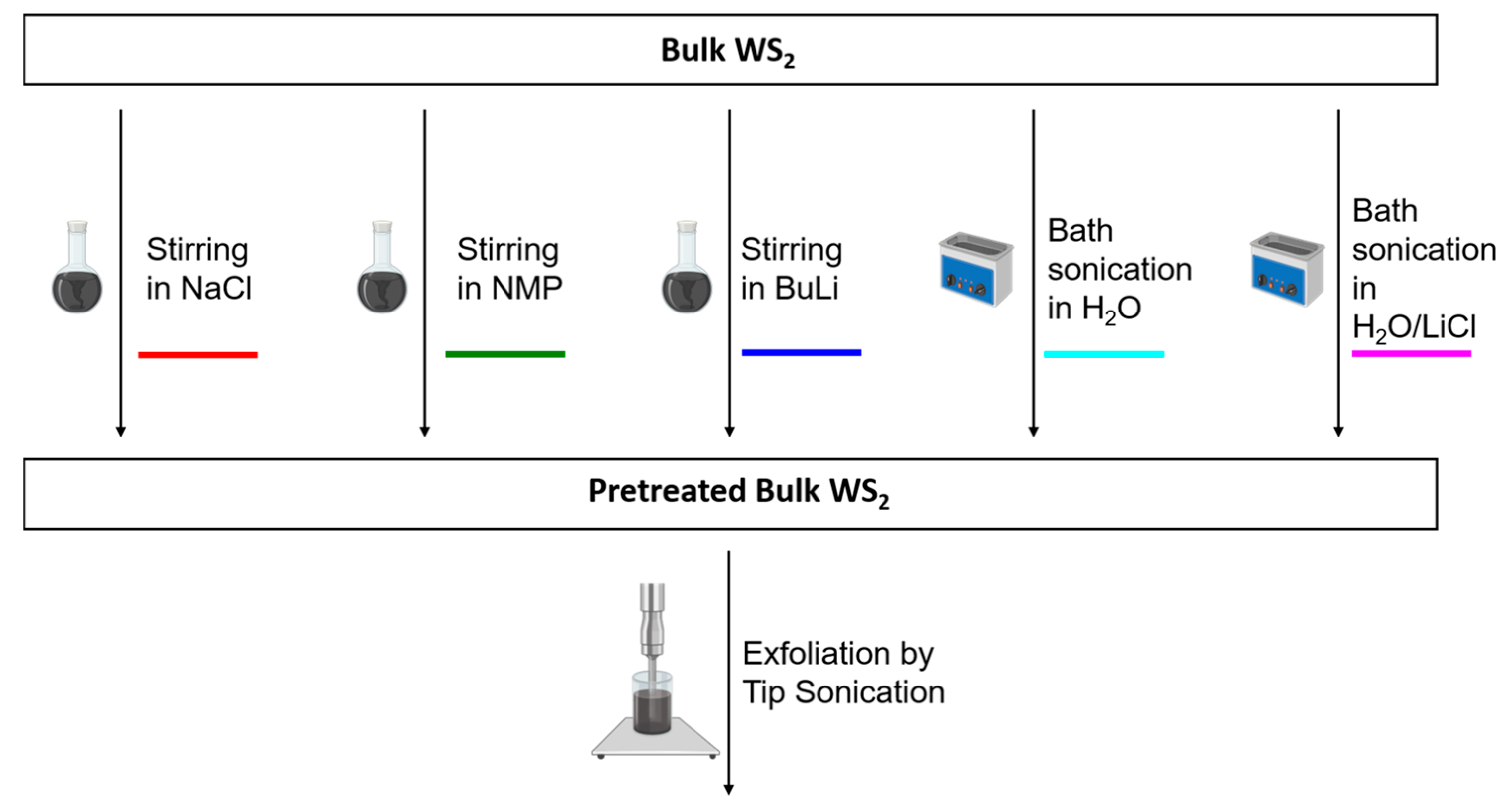
Nanomaterials | Free Full-Text | Impact of Pretreatment of the Bulk Starting Material on the Efficiency of Liquid Phase Exfoliation of WS2 | HTML
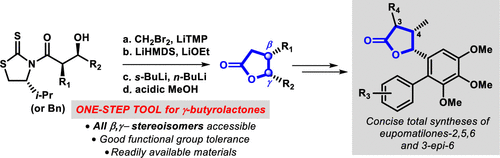
Synthesis of γ-Lactones via the Kowalski Homologation Reaction: Protecting-Group-Free Divergent Total Syntheses of Eupomatilones-2,5,6, and 3-epi-Eupomatilone-6,Organic Letters - X-MOL
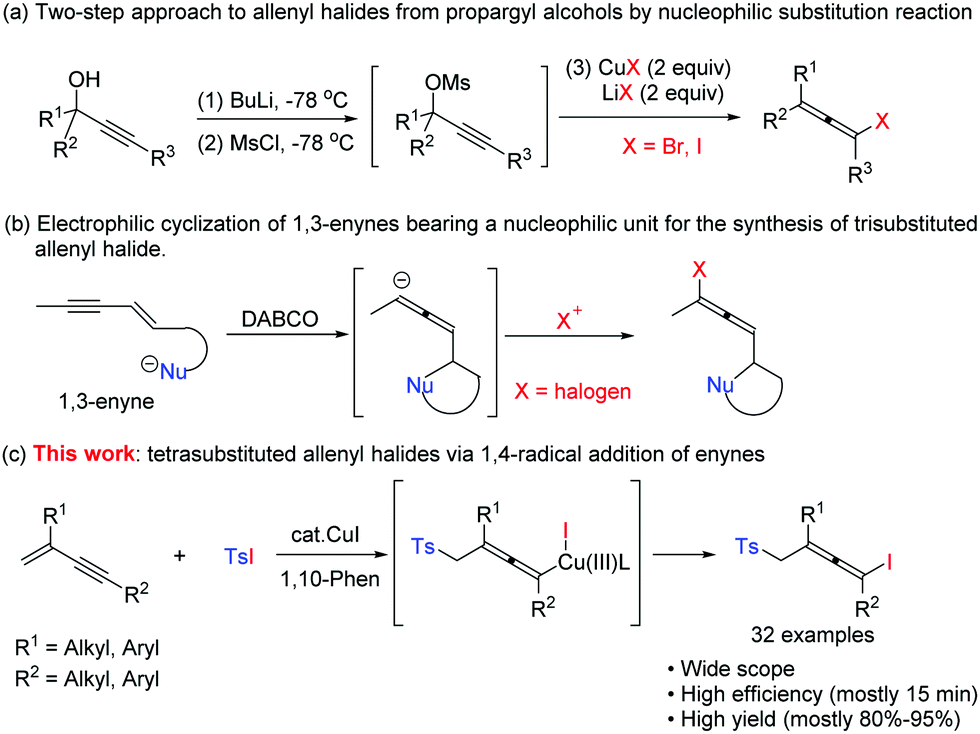
Copper-catalyzed radical approach to allenyl iodides - Chemical Communications (RSC Publishing) DOI:10.1039/C9CC05853B

Synthesis, Reaction, and Recycle of Fluorous Palladium Catalysts for an Asymmetric Allylic Alkylation without Using Fluorous Solvents | The Journal of Organic Chemistry
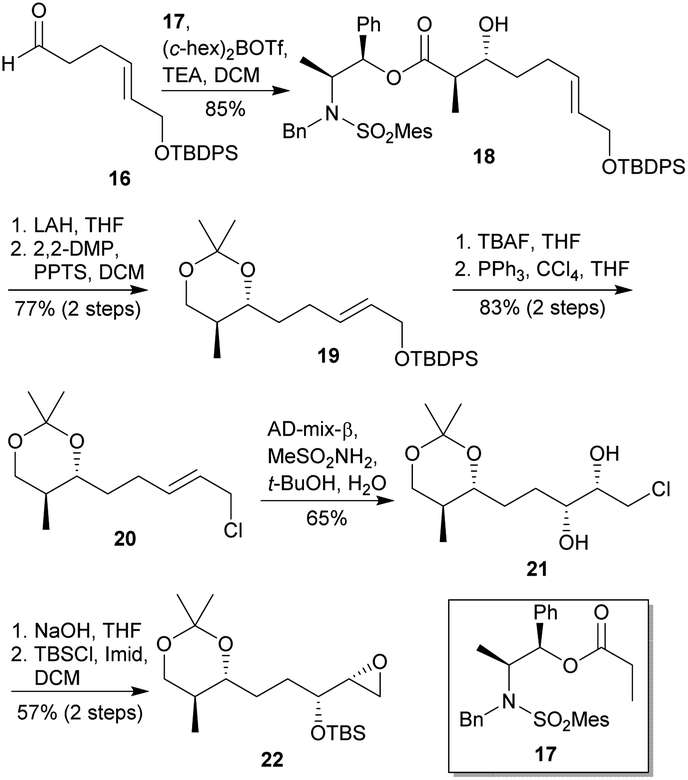
Synthetic efforts on the road to marine natural products bearing 4- O -2,3,4,6-tetrasubstituted THPs: an update - RSC Advances (RSC Publishing) DOI:10.1039/D0RA10755G

Intensification of Continuous Ortho-Lithiation at Ambient Conditions—Process Understanding and Assessment of Sustainability Benefits,Organic Process Research & Development - X-MOL

Generation and Electrophile Trapping of N -Boc-2-lithio-2-azetine: Synthesis of 2-Substituted 2-Azetines – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.
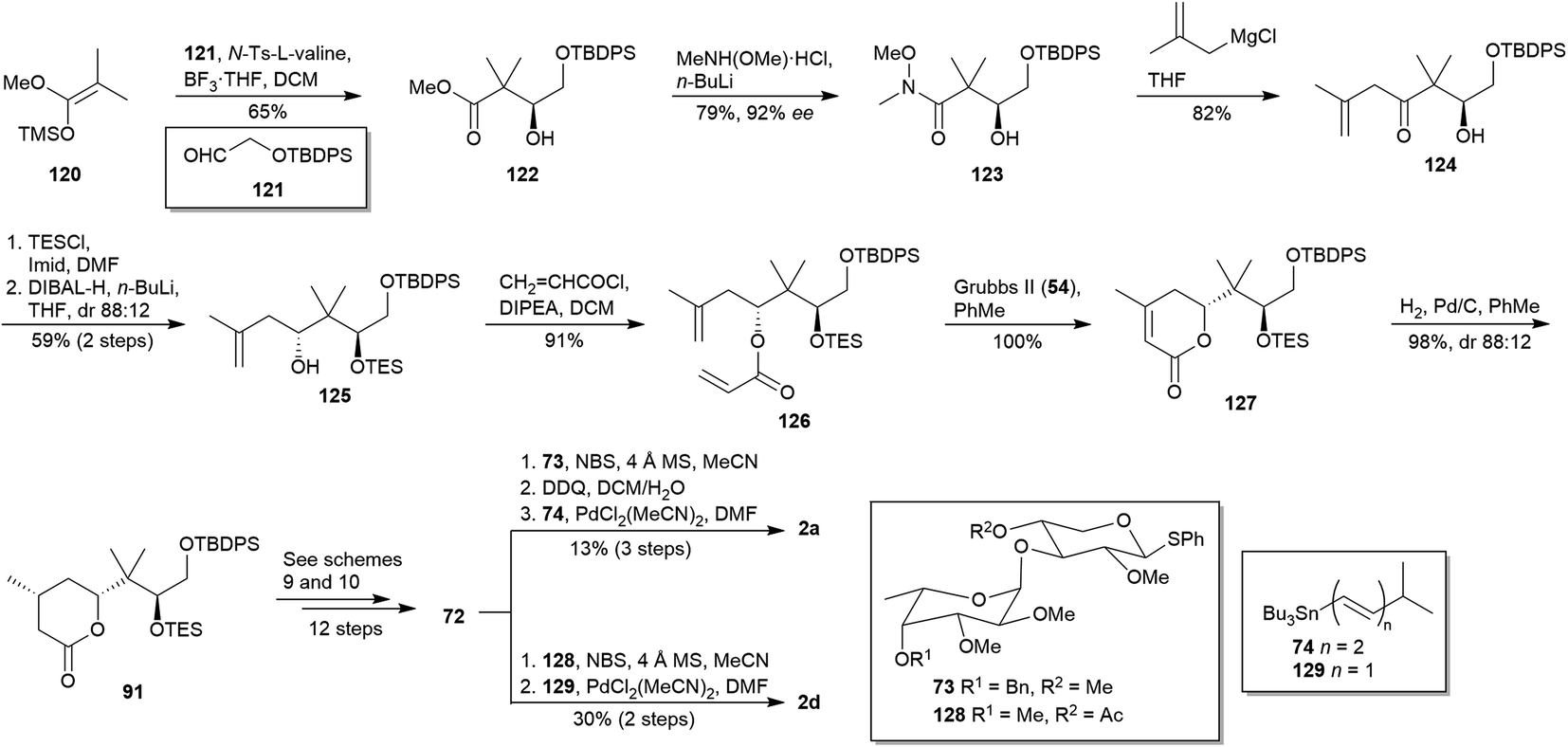
Synthetic efforts on the road to marine natural products bearing 4- O -2,3,4,6-tetrasubstituted THPs: an update - RSC Advances (RSC Publishing) DOI:10.1039/D0RA10755G
![PM3 optimized structures of [n-BuLi] 4 ·THF 4 and [n-BuLi] 2 ·THF 4.... | Download Scientific Diagram PM3 optimized structures of [n-BuLi] 4 ·THF 4 and [n-BuLi] 2 ·THF 4.... | Download Scientific Diagram](https://www.researchgate.net/profile/Stefan-Berger-10/publication/226627566/figure/fig2/AS:667612778356749@1536182698615/PM3-optimized-structures-of-n-BuLi-4-THF-4-and-n-BuLi-2-THF-4-Reprinted-with.png)
PM3 optimized structures of [n-BuLi] 4 ·THF 4 and [n-BuLi] 2 ·THF 4.... | Download Scientific Diagram
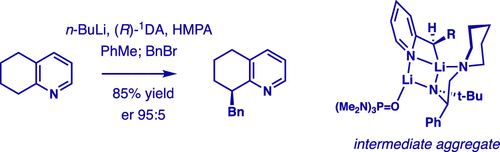
Enantioselective Alkylation of 2-Alkylpyridines Controlled by Organolithium Aggregation.,Journal of the American Chemical Society - X-MOL

Halogen–Lithium Exchange Reaction Using an Integrated Glass Microfluidic Device: An Optimized Synthetic Approach,Organic Process Research & Development - X-MOL

Five-Step Total Synthesis of (±)-Aspidospermidine by a Lactam Strategy via an Azomethine Ylide | Organic Letters