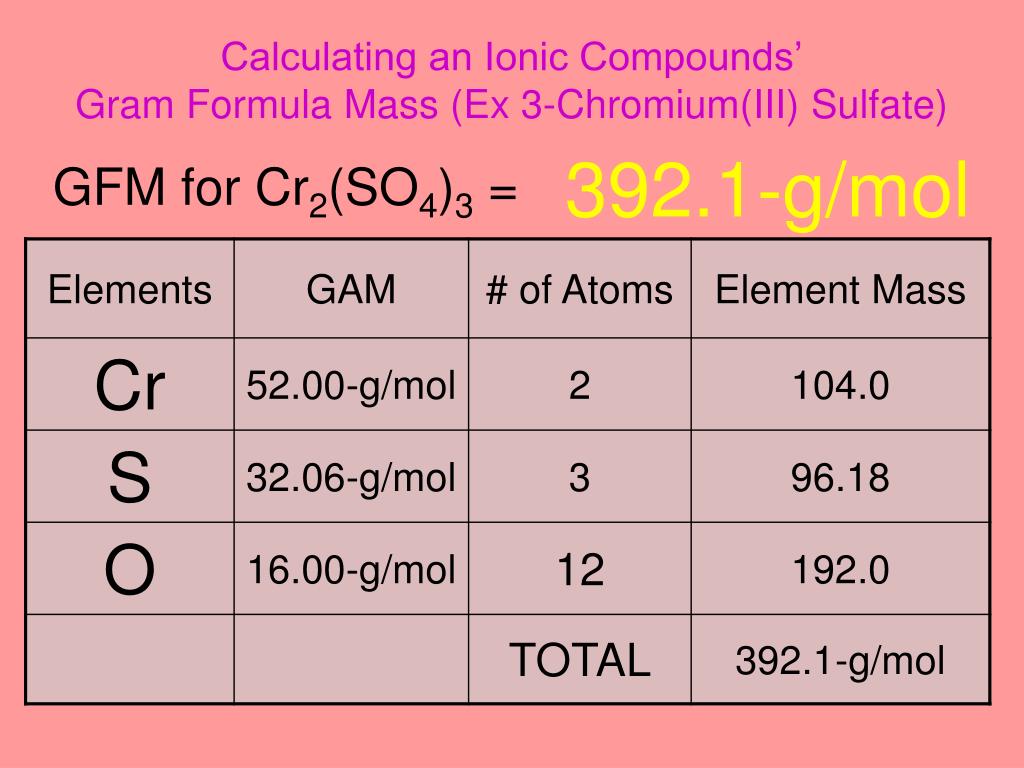
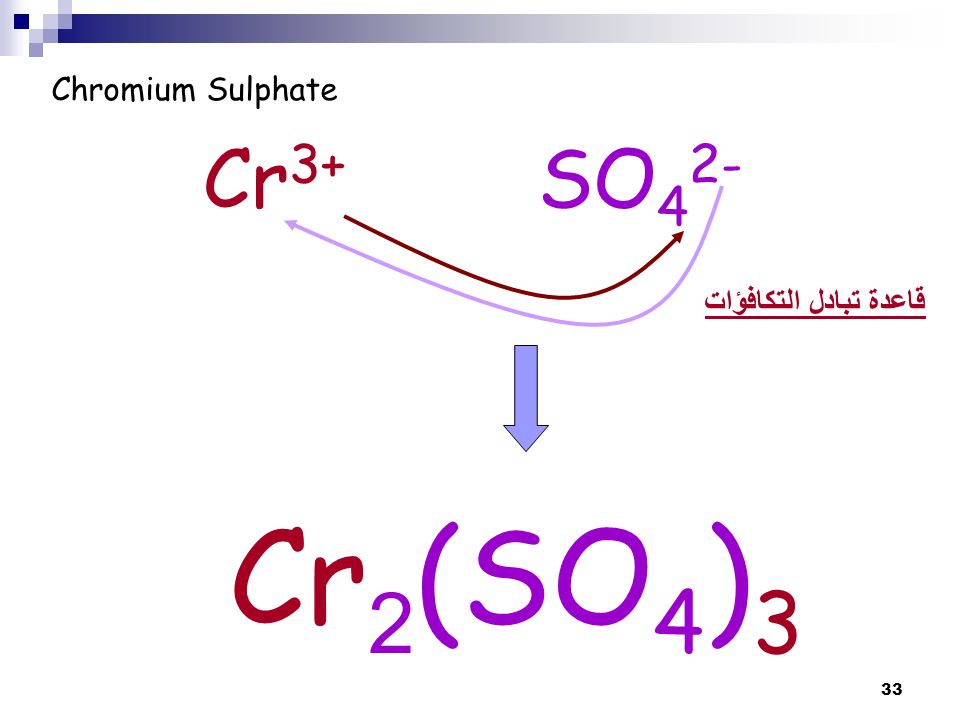
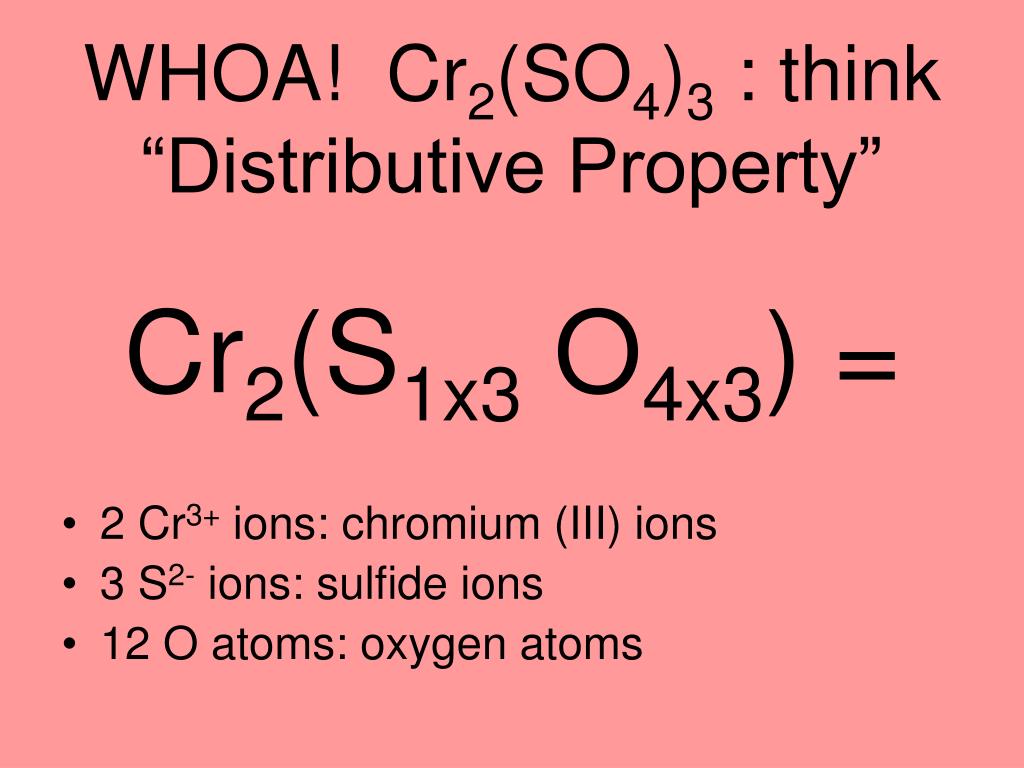
Balance the following equations by oxidation number method 1. K2Cr2O7 + KI + H2SO2 → K2SO4 + Cr2(SO4)3 + I2 + H2O - Sarthaks eConnect | Largest Online Education Community
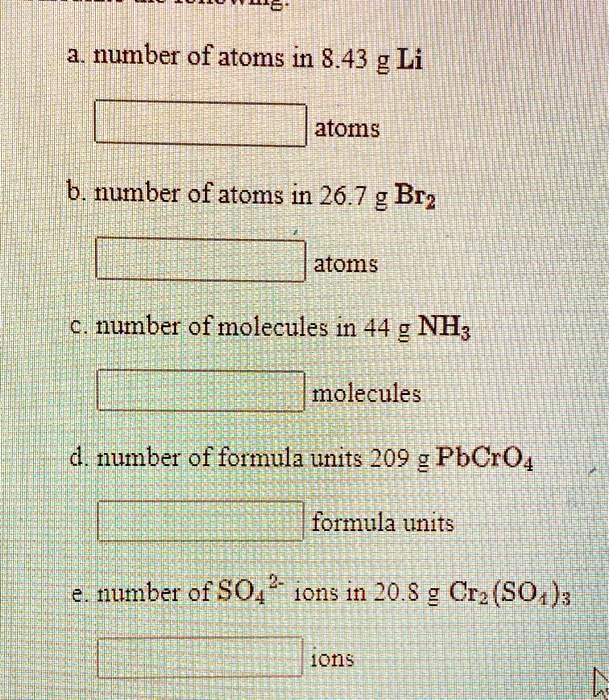
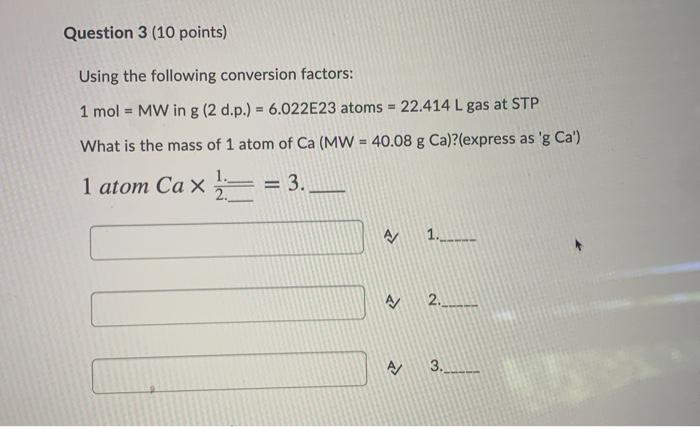
SOLVED:number of atoms in 8.43 g Li atoms b number of atoms in 26.7 g Br? atoms number of molecules in 44 g NH; molecules d. number of formula units 209 g
Balance the following equations by oxidation number method 1. K2Cr2O7 + KI + H2SO2 → K2SO4 + Cr2(SO4)3 + I2 + H2O - Sarthaks eConnect | Largest Online Education Community
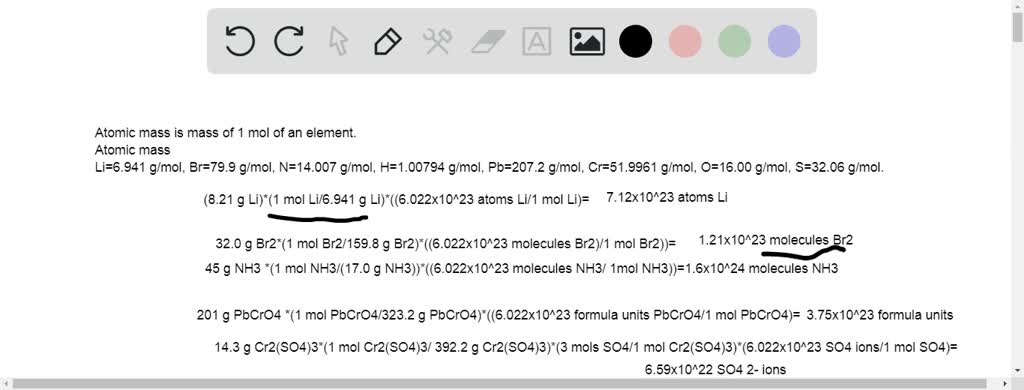
SOLVED:Calculate the following. number of atoms in 8.21 \mathrm{~g} \mathrm{Li} number of atoms in 32.0 \mathrm{~g} \mathrm{Br}_{2} number of molecules in 45 \mathrm{~g} \mathrm{NH}_{3} number of formula units in 201 \mathrm{~g} \mathrm{PbCrO}_{4}