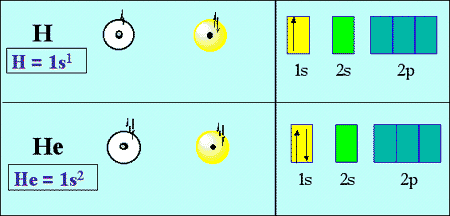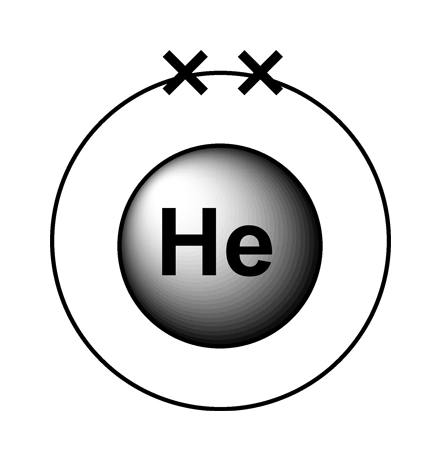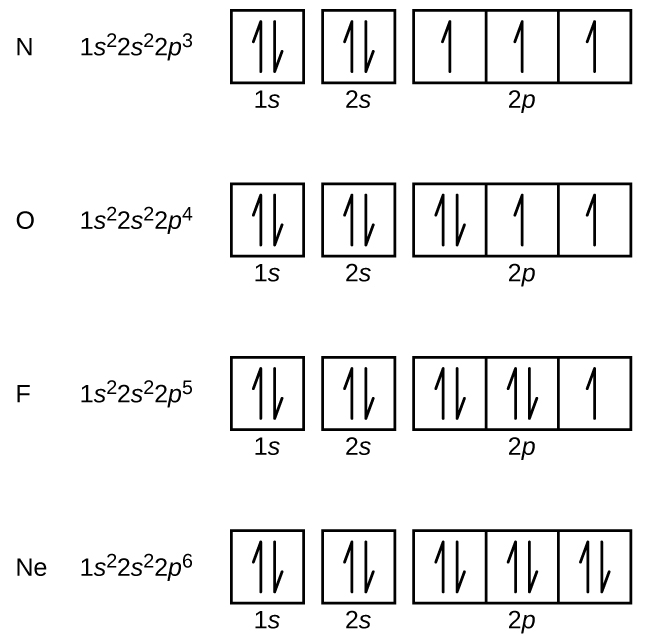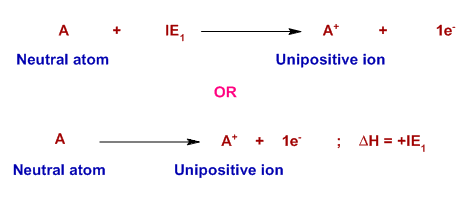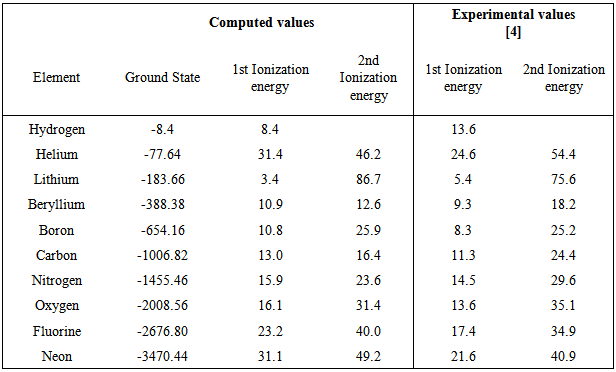
Computation of the First and Second Ionization Energies of the First Ten Elements of the Periodic Table Using a Modified Hartree-Fock Approximation Code
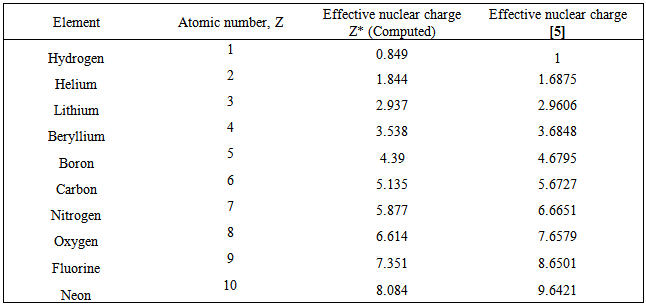
Computation of the First and Second Ionization Energies of the First Ten Elements of the Periodic Table Using a Modified Hartree-Fock Approximation Code

Computation of the First and Second Ionization Energies of the First Ten Elements of the Periodic Table Using a Modified Hartree-Fock Approximation Code